Anzeige
Meldung des Tages: Eine der spannendsten Gold-Stories 2026 steht ganz am Anfang
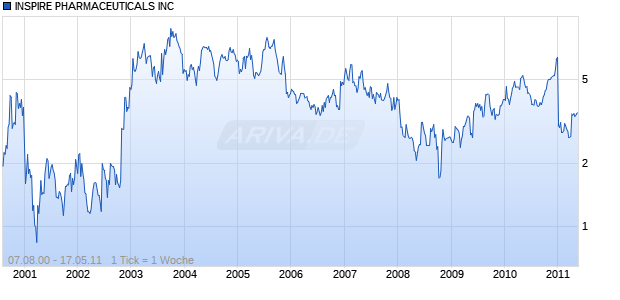
INSPIRE PHARMACEUTICALS INC
Aktie
WKN: 940543 ISIN: US4577331030
Keine aktuellen Kursdaten verfügbar
Depot/Watchlist
Dieses Wertpapier ist nicht mehr handelbar.
- Push
- Intraday
- 1W
- 3M
- 6M
- 1J
- 5J
- Gesamt
Werbung
Mehr Nachrichten kostenlos abonnieren
E-Mail-Adresse
Bitte überprüfe deine die E-Mail-Adresse.
Benachrichtigungen von ARIVA.DE
(Mit der Bestellung akzeptierst du die Datenschutzhinweise)
(Mit der Bestellung akzeptierst du die Datenschutzhinweise)

Vielen Dank, dass du dich für unseren Newsletter angemeldet hast. Du erhältst in Kürze eine E-Mail mit einem Aktivierungslink.
Leider können wir deine Anfrage auf diesem Weg nicht entgegennehmen.
Bitte schreibe uns an: portal.support@ariva.de
Bitte schreibe uns an: portal.support@ariva.de
Termine
Keine Termine bekannt.
Prognose & Kursziel
Keine aktuellen Prognosen oder Kursziele bekannt.
Stammdaten
| Aktientyp | Stammaktie |
Community-Beiträge zu INSPIRE PHARMACEUTICALS INC
martin30sm
Zumindest eine Gegenbewegung sollte es heute geben
4 Dollar sollten kurzfristig möglich sein
martin30sm
Schon heftig dieser Absturz!
Langfristig ev. Chance?
Eskimato
Das war ja mal ne Verkaufsempfehlung.
Von 16 runter auf 11, soweit so gut.
Mal sehen wie es nach den News weitergeht.
Gruss E.
Inspire Pharmaceuticals Submits New Drug Application For Diquafosol For The Treatment of Dry Eye
FRIDAY, JUNE 27, 2003 11:45 AM
- PR Newswire
DURHAM, N.C., Jun 27, 2003 /PRNewswire-FirstCall via COMTEX/ -- Inspire Pharmaceuticals, Inc. (ISPH) today announced the submission of a New Drug Application (NDA) to the U.S. Food and Drug Administration (FDA) for approval to market diquafosol tetrasodium (INS365) eye drops in a 2% preservative-free solution. Diquafosol represents a new approach to the treatment of dry eye. By stimulating P2Y2 receptors located on the ocular surface and inner lining of the eyelid, diquafosol enhances the secretion of water, salt, mucin and lipids -- key components of natural tears. This increase in the major components of the tear film is believed to result in improved tear volume and tear composition.
The diquafosol NDA submission includes data from one Phase II and two Phase III studies involving over 1,200 dry eye patients. Key data from the studies will be presented at the European Association for Vision and Eye Research (EVER) meeting in Alicante, Spain in October 2003.
"This NDA, Inspire's first, is a major achievement and is the result of nearly six years of focused and dedicated effort on the part of our employees, with valuable support from our partners and guidance from the FDA," stated Christy L. Shaffer, Ph.D., CEO of Inspire. "This milestone marks an important transition for our business as we begin building commercial capabilities."
The NDA submission triggers a milestone payment from Inspire's partner Allergan.
The FDA customarily accepts or refuses to file NDAs and designates review status within 60 days of submission.
About Dry Eye
Dry eye is a painful, burning and irritating condition involving abnormalities and deficiencies in the tear film due to a variety of causes. It affects approximately 10 million North Americans and is one of the most frequent patient complaints reported to ophthalmologists and optometrists. The majority of dry eye patients rely on artificial tears to relieve symptoms. Allergan's Restasis(TM) is the first and only pharmacologically active prescription product approved for patients with chronic dry eye disease.
About Inspire
Inspire Pharmaceuticals, Inc. discovers and develops new drugs to treat diseases characterized by deficiencies in the body's innate defense mechanisms of mucosal hydration and mucociliary clearance, as well as other non-mucosal disorders. Mucosal defense mechanisms are the natural way that the body defends its mucosal surfaces against dust, pollutants, bacteria and viruses. Inspire's lead product candidates target ophthalmic and respiratory diseases with inadequate current treatments and which represent large therapeutic market opportunities. Inspire has development and commercialization alliances with Allergan, Inc., Santen Pharmaceutical Co., Ltd. and Kirin Brewery Co., Ltd., and has a collaboration with Cystic Fibrosis Foundation Therapeutics, Inc. Inspire's products are based on proprietary technology relating to P2Y2 receptors and to non-P2Y2 receptors that show therapeutic promise, including P2Y12.
Forward-Looking Statements
The forward-looking statements in this news release relating to management's expectations and beliefs are based on preliminary information and management assumptions. Such forward-looking statements are subject to a wide range of risks and uncertainties that could cause results to differ in material respects, including those relating to product development, revenue and earnings expectations, intellectual property rights and litigation, competitive products, results of clinical trials, the need for additional research and testing, delays in manufacturing, funding and the timing and content of decisions made by regulatory authorities, including the United States Food and Drug Administration. Further information regarding factors that could affect Inspire's results is included in Inspire's filings with the Securities and Exchange Commission. Inspire undertakes no obligation to publicly release the results of any revisions to these forward-looking statements that may be made to reflect events or circumstances after the date hereof.
Eskimato
Aber hier die Party verlassen.
Liest sich nicht so gut,
Inspire Pharmaceuticals (ISPH: news, chart, profile) lost more than 14 percent after the company said a recent trial of its treatment for perennial allergic rhinitis was well-tolerated but it missed its primary endpoint of "significantly reducing the total nasal symptom score over the 28-day treatment period versus placebo." The company plans to conduct secondary analyses to determine future plans for the INS37217 intranasal compound.
Gruss E.

Jetzt anmelden und diskutieren
Registrieren
Login
Zum Thread wechseln Häufig gestellte Fragen zur INSPIRE PHARMACEUTICALS INC Aktie und zum INSPIRE PHARMACEUTICALS INC Kurs
Am 09.02.2005 gab es einen Split im Verhältnis 1:2.
Am 09.02.2005 gab es einen Split im Verhältnis 1:2.
Nein, INSPIRE PHARMACEUTICALS INC zahlt keine Dividenden.