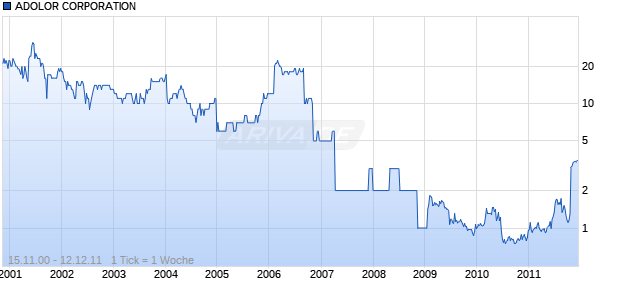
ADOLOR CORPORATION
- Push
- Intraday
- 1W
- 3M
- 6M
- 1J
- 5J
- Gesamt
Mehr Nachrichten kostenlos abonnieren
(Mit der Bestellung akzeptierst du die Datenschutzhinweise)

Bitte schreibe uns an: portal.support@ariva.de
Termine
Prognose & Kursziel
Stammdaten
| Aktientyp | Stammaktie |
Community-Beiträge zu ADOLOR CORPORATION
 Im Prinzip verdammt zu steigen. Im Prinzip........
Im Prinzip verdammt zu steigen. Im Prinzip........
Adolor Provides Update on Entereg(R) (alvimopan) OBD Program Thursday July 3, 7:30 am ET FDA Lifts Clinical Hold on OBD IND
EXTON, Pa.--(BUSINESS WIRE)--Adolor Corporation (Nasdaq: ADLR - News) today issued an update on the Entereg® (alvimopan) Program for chronic opioid bowel dysfunction (OBD), under development in collaboration with GlaxoSmithKline (NYSE: GSK - News). ADVERTISEMENT The U. S. Food and Drug Administration (FDA) has concluded that clinical investigations relating to alvimopan in OBD may now proceed, and has therefore lifted the clinical hold on the OBD Investigational New Drug Application. “After a productive meeting and dialogue with FDA, we are very pleased to see the clinical hold lifted,” said Michael R. Dougherty, president and chief executive officer of Adolor. “There remains a large, unmet need for treatment options for the many patients suffering from this debilitating condition.” Adolor understands that GSK is evaluating all options relating to the OBD Program, including whether to proceed with its involvement with the Program. The April 2002 Collaboration Agreement between Adolor and GSK provides that GSK may terminate the Agreement with respect to the OBD product, returning rights to the OBD product to Adolor, while retaining its rights to the postoperative ileus (POI) product. Michael R. Dougherty said, “Should GSK determine to discontinue their involvement with the OBD Program, Adolor would expect to submit for review by FDA a protocol for an additional study in this indication.” GSK and Adolor are actively engaged in the commercialization of the recently approved Entereg for POI for bowel resection surgeries.
Gaaaaaaaaaaaaaaaaaaaaaaaaannnnnz wichtig!
Nun hat Adolor und Glaxo Smith Kline die Zulassung von Entereg für Post Operative Illeus (POI) bekommen, aber im Kurs von ADLR nur leichte Bewegung nordwärts. Da steckt noch viel neg. Stimmung in die Knochen, die sich ADLR nach den Studien OBD eingefagen hat. Glaxo hat aber in der Zulassung gesagt, dass "A causal relationship with alvimopan has not been established."
Im Kontext.
There were more reports of myocardial infarctions in patients treated with alvimopan 0.5 mg twice daily compared with placebo-treated patients in a 12-month study of patients being treated with opioids for chronic pain. This imbalance has not been observed in studies in patients undergoing bowel resection surgery who have received alvimopan 12 mg twice daily for up to 7 days. A causal relationship with alvimopan has not been established.
Das sollte die Wahrscheinlichkeit massiv erhöhen, dass Entereg auch auf dem lukrativeren OBD Markt zuglassen wird. Eines sonnigen Tages jedenfalls.