https://www.genprex.com/investors/sec-filings/
https://www.genprex.com/news/category/press-releases/
Genprex mit ihrem Hauptproduktkandidat Onkoprex in Phase2
Combining Oncoprex™ With Tarceva®
A phase I/II clinical trial is underway evaluating intravenous Oncoprex in combination with Tarceva® (erlotinib) in stage IIIB/IV lung cancer patients without an activating EGFR mutation and in patients with an activating EGFR mutation whose cancer has progressed on erlotinib therapy.
We believe that the results from the ongoing Phase II trial to date are encouraging. Out of 10 patients, 9 had received 2 or more cycles and were therefore evaluable for response. Four patients had tumor regression. The median duration of response is 3 months. The disease control rate (CR+PR+SD > 8weeks) was 78%, which substantially exceeds the 7% response rate (with no CRs) and 58% disease control rate reported for the LUX-Lung 1 trial, a clinical trial of afatinib in a comparable group of patients.
One patient in the Phase II combination trial had a Complete Response
https://www.genprex.com/clinical-trials/erlotinib-combination/
Studienende :
| Estimated Primary Completion Date : | March 2020 |
| Estimated Study Completion Date : | July 2020 |
Zu bedenken bei den Ansprechraten ist die weit vortgeschrittene Krankheit!
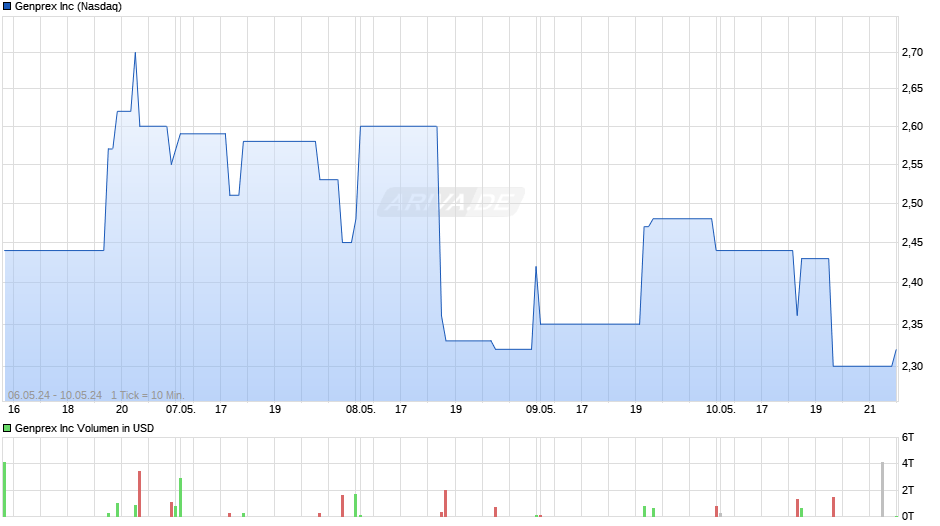
Shares out: 15,1Mio
Über 50% schon vergeben! SEC Filings vom 15.2.19