Anzeige
Meldung des Tages: SGS liefert den Ritterschlag: Weltklasse-Tests machen aus einem Explorer plötzlich ein Industrie-Asset
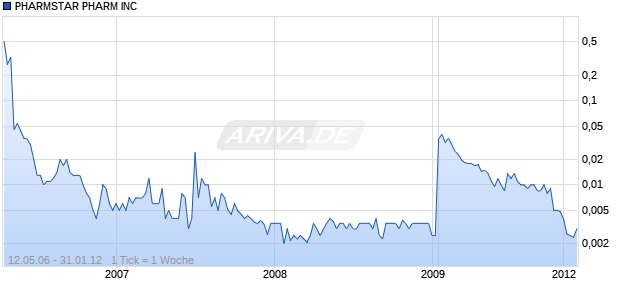
PHARMSTAR PHARM INC
Aktie
WKN: 917797 ISIN: US71713J1079
Keine aktuellen Kursdaten verfügbar
Depot/Watchlist
Dieses Wertpapier ist nicht mehr handelbar.
Marktkapitalisierung *
-
Streubesitz
-
KGV
-
Index-Zuordnung
-
- Push
- Intraday
- 1W
- 3M
- 6M
- 1J
- 5J
- Gesamt
Werbung
Mehr Nachrichten kostenlos abonnieren
E-Mail-Adresse
Bitte überprüfe deine die E-Mail-Adresse.
Benachrichtigungen von ARIVA.DE
(Mit der Bestellung akzeptierst du die Datenschutzhinweise)
(Mit der Bestellung akzeptierst du die Datenschutzhinweise)

Vielen Dank, dass du dich für unseren Newsletter angemeldet hast. Du erhältst in Kürze eine E-Mail mit einem Aktivierungslink.
Leider können wir deine Anfrage auf diesem Weg nicht entgegennehmen.
Bitte schreibe uns an: portal.support@ariva.de
Bitte schreibe uns an: portal.support@ariva.de
Termine
Keine Termine bekannt.
Prognose & Kursziel
Keine aktuellen Prognosen oder Kursziele bekannt.
Stammdaten
| Aktientyp | Stammaktie |
Community-Beiträge zu PHARMSTAR PHARM INC
Kicky
gibts nicht mehr seit 13 Mai 2005
PharmaNetics, Inc. (PHAR.OB) announced today that it has filed a Form 15 to deregister its common stock and suspend its reporting obligations under the Securities Exchange Act of 1934, effective immediately upon such filing on May 13, 2005. As a result of the filing of the Form 15, the Company's obligation to file with the SEC virtually all reports and forms, including its quarterly and annual reports, has terminated. In addition, the common stock of the Company will no longer be quoted on the OTC Bulletin Board.
(hättest ja wirklich mal auf den Link und News klicken können !wer zockt auch mit sonem Wert!)
bullorbear
weiß jemand bescheid?
hallo zusammen!
wer kennt sich denn mit diesem wert aus?
gebt mir mal bitte informationen, die mich
weiterbringen ....
lg, bull
Kicky
up für geldschneider o. T.
Kicky
hat im März 2004 mit Business aufgehört
den Vertrag mit Bayer auslaufen lassen,alle Leute entlassen bis auf den CEO,der noch immer versucht,Lizenz(intellectual Property) und Gebäude zu verkaufen
PharmaNetics, Inc. (the “Company” or “PharmaNetics”), is a holding company incorporated in North Carolina in 1998 as the parent company of Cardiovascular Diagnostics, Inc. Cardiovascular Diagnostics, Inc. (“CVDI”) was incorporated in 1985 and was the sole operating subsidiary of PharmaNetics until PharmaNetics ceased substantially all of its operations in March 2004.
Prior to ceasing substantially all of its operations in March 2004 (see “Recent Developments” below), PharmaNetics developed, manufactured and marketed rapid diagnostics to dose, manage and screen patients on drugs affecting coagulation. The Company’s products are a proprietary analyzer and dry chemistry tests and controls, known as the Thrombolytic Assessment System, or TAS, that provide a physician, at the point of patient care, information that can affect therapy. The Company’s tests were and can be used in the treatment of a variety of adverse conditions caused by abnormal blood clotting in different areas of the body, including angina, heart attack, stroke, deep vein thrombosis and pulmonary and arterial emboli.
In November 2003, the Company filed a lawsuit in the eastern district of North Carolina against Aventis Pharmaceuticals, Inc. (“Aventis”). The Company, in cooperation with Aventis, has developed a rapid bedside test, known as the Enox test, that the Company believes enhances the way Lovenox®, a popular anti-blood clotting drug marketed by Aventis, currently is managed. The Company believes the test has the potential to facilitate the drug’s use in patients in the cardiac community who stand to benefit from its use. Aventis collaborated with the Company in a multi-million dollar project in which it made milestone payments to the Company to develop and co-promote the test together with Lovenox for targeted patient populations. The lawsuit alleges that Aventis has engaged in false and misleading advertising of Lovenox, which damaged the Company’s efforts to market and sell the Enox test card.
und in diesem Prozess wurde zwar bestätigt dass die Angaben von Aventis irreführensd seien,aber Aventis wurde nicht verurteilt
As of the date of this filing, pursuant to notice duly given, the Company has allowed the distribution agreement with Bayer to expire and has terminated the Enox contract sales and technical service force. Because no buyer of the manufacturing operations or intellectual property has yet emerged, effective by the end of March 2004, the Company has ceased developing, producing and selling all of its products and plans to terminate substantially all remaining employees except the chief executive officer (CEO). The Company plans to retain the CEO to manage the Aventis litigation until it is completed or settled and to continue to seek a buyer of the operations, manufacturing assets and intellectual property
http://www.pinksheets.com/quote/filings.jsp?symbol=PHAR
hier 8K v.5.5. und 10K v.14.4.
da hat sich wohl jemand von dem damals engagierten Finanzberater Davenport & CO einlullen lassen,als er diese Aktie empfahl ggg
Jetzt anmelden und diskutieren
Registrieren
Login
Zum Thread wechseln Häufig gestellte Fragen zur PHARMSTAR PHARM INC Aktie und zum PHARMSTAR PHARM INC Kurs
Nein, PHARMSTAR PHARM INC zahlt keine Dividenden.