Anzeige
Meldung des Tages: Analyst sieht bei dieser Food-Tech-Aktie jetzt rund 100 % Kurspotenzial
Neuester,
zuletzt geles. Beitrag
Antworten | Hot-Stocks-Forum Übersicht Zurück
Weiter
Zurück
Weiter
... 7 8 9 10 12 13 14 15 ...
Antworten | Hot-Stocks-Forum Übersicht
... 7 8 9 10 12 13 14 15 ...
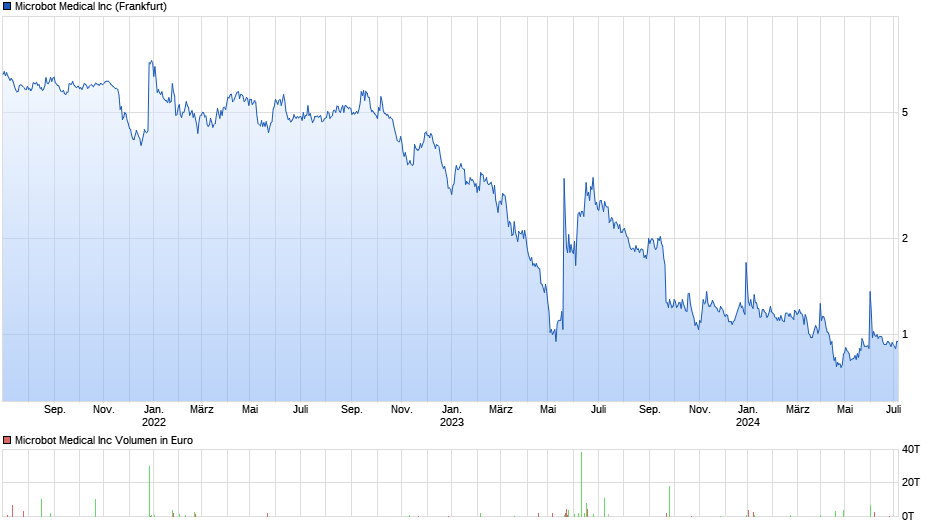
Microbot Medical übernimmt Stemcells - Neustart
Beiträge:
393
Zugriffe: 131.852 / Heute: 4
Zugriffe: 131.852 / Heute: 4
|
Werbung
Entdecke die beliebtesten ETFs von SPDR
dass der Kurs so nachgibt nach all den guten News.....entäuschend....klar das Marktumfeld ist schlecht, aber trotzdem hätte der 1 USD halten sollen/können...
Dieses Video wird aufgrund Ihrer Datenschutzeinstellungen nicht abgespielt. Wenn Sie dieses Video betrachten möchten, geben Sie bitte hier die Einwilligung, dass wir Ihnen Youtube-Videos anzeigen dürfen.
und nicht nur MBOT. Dirk Müller zum Flash-Crash!
Quelle: https://www.youtube.com/watch?v=LGY33vQ0JLE
Gelöschter Beitrag.
Einblenden
»
#255
www.slideshare.net/MicroBotVideo/...botics?platform=hootsuite
Microbot Medical’s CEO to Speak as an Industry Expert at the YJP CEO Healthcare Symposium
Quelle: globenewswire.com/news-release/2018/02/13/...e-Symposium.html
Quelle: globenewswire.com/news-release/2018/02/13/...e-Symposium.html
Gelöschter Beitrag.
Einblenden
»
#258
also m.E. sollte MBOT bis 2019 genug Cash haben, zudem staatlich gefördert durch Israel. Im Jahr 2018 sollte der FDA Antrag eingereicht werden. m.M.
"Steht eigentlich noch immer eine Kapitalerhöhung im Raum?"
Wann hast Du denn diese Information aufgeschnappt? Ich bin in MBOT seit September vergangenen Jahres investiert und habe verdammt viel Recherche betrieben. Solche Aussagen wurden bei Stocktwits von einigen Bashern von Zeit zu Zeit gestreut - ohne sie konkret zu belegen.
Aus KEINEM Quartalsbericht oder dem Townhall Meeting vor einigen Monaten ging auch nur der Hauch einer entsprechenden Andeutung hervor. Stattdessen wurde bei der Darstellung der finanziellen Situation des Unternehmens immer wieder darauf hingewiesen, dass die MBOT bis 2019 abgesichert ist.
Ich würde dir vor diesem Hintergrund hier einige Posts nach oben zu scrollen. Da findest du die Präsentation des Townhall Meetings und damit auch die Angaben zur finanziellen Situation.
Bevor Du also solche Aussagen in den Raum stellst, solltest Du zunächst besser recherchieren!
Wann hast Du denn diese Information aufgeschnappt? Ich bin in MBOT seit September vergangenen Jahres investiert und habe verdammt viel Recherche betrieben. Solche Aussagen wurden bei Stocktwits von einigen Bashern von Zeit zu Zeit gestreut - ohne sie konkret zu belegen.
Aus KEINEM Quartalsbericht oder dem Townhall Meeting vor einigen Monaten ging auch nur der Hauch einer entsprechenden Andeutung hervor. Stattdessen wurde bei der Darstellung der finanziellen Situation des Unternehmens immer wieder darauf hingewiesen, dass die MBOT bis 2019 abgesichert ist.
Ich würde dir vor diesem Hintergrund hier einige Posts nach oben zu scrollen. Da findest du die Präsentation des Townhall Meetings und damit auch die Angaben zur finanziellen Situation.
Bevor Du also solche Aussagen in den Raum stellst, solltest Du zunächst besser recherchieren!
Sorry, hatte einen gedanklichen Dreher. 😶
Aber unabhängig von einer Kapitalerhöhung... glaubt Ihr tatsächlich, der Cash-Bestand reicht bis 2019. Finde die Prognose echt gewagt. Auch wenn eine Kapitalerhöhung aktuell nicht im Raum steht, spätestens zum Jahresende wird diese sicherlich ein Thema. Eine Kapitalerhöhung finde ich auch nicht schlimm.
Aber unabhängig von einer Kapitalerhöhung... glaubt Ihr tatsächlich, der Cash-Bestand reicht bis 2019. Finde die Prognose echt gewagt. Auch wenn eine Kapitalerhöhung aktuell nicht im Raum steht, spätestens zum Jahresende wird diese sicherlich ein Thema. Eine Kapitalerhöhung finde ich auch nicht schlimm.
Die aktuellen Verkäufe deutet m. E. darauf hin, dass manche Leute schon mehr wissen und mit einem Kursrückgang rechnen!
Unter 0,30 steige ich wieder ein.
Unter 0,30 steige ich wieder ein.
www.microbotmedical.com/investors/sec-filings/
10-K vom 2.4.2018
"Microbot has completed the development of an SCS prototype and is currently completing the safety testing, general proof of concept testing and performance testing for the device, which Microbot began in mid-2013. Microbot had a pre-submission meeting with the FDA in mid-2014. On January 27, 2017, Microbot entered into a research agreement with The Washington University in St. Louis to develop the protocol for and to execute the necessary animal study to determine the effectiveness of the Microbots SCS prototype. The initial research was completed in 2017, and a comprehensive study is expected to be completed in 2018. Upon the completion of animal studies, Microbot may conduct clinical trials if they are requested by the FDA or if Microbot decides that the data from such trials would improve the marketability of the product candidate. Microbot believes that the study results of its first generation SCS device should be submitted to the FDA by late 2018." (S. 3).
"Microbot intends to focus its marketing and sales efforts initially on pursuing collaborations with global medical device companies that have established sales and distribution networks. Microbot will seek to enter collaborations and partnerships with strategic players that offer synergies with Microbots product candidates and expertise." (S. 4).
"Microbots SCS device is universal (meaning that it is designed to be attachable to any valve on the market); therefore, Microbots initial go-to-market strategy is the development of strategic partnerships with leading global medical device companies with ready sales and distribution channels." (S. 4).
"Several academic research groups, such as at the New Jersey Institute of Technology, are currently researching sensing and obstruction-resistant catheter designs, and the Smart Sensors and Integrated Microsystems (SSIM) Program at Wayne State University has publicized that it is engaging in smart shunt development activity. However, based on its knowledge of the patented technologies, Microbot believes that these technologies are still early in the research and development cycle. The SCS also faces non-direct competition from Aqueduct Neurosciences, Inc., which is developing a non-shunt, electro-mechanical technology platform to control the draining of cerebrospinal fluid.
Microbot does not expect its SCS device to directly compete against shunt systems currently available in the market." (!!! / S.4).
"Microbot has not yet established a sales, marketing or product distribution infrastructure for its product candidates, which are still in development stages. Microbot plans to access the U.S. markets for hydrocephalus, NPH, and colonoscopy with its initial device offerings through strategic partnerships but may develop its own focused, specialized sales force or distribution channels once it has several commercialized products in its portfolio." (S. 7).
"Microbot is a development-stage medical device company and currently generates no revenue from product sales, and may never be able to commercialize SCS, TipCAT or other future product candidates, including the potential acquisition of technology from CardioSert Ltd. Microbot does not currently have the required approvals or clearances to market or test in humans SCS, TipCAT or any other future product candidates and Microbot may never receive them." [...] "Microbots business depends on the success of the SCS and the TipCAT, both of which are still in pre-clinical development. If Microbot is unable to obtain regulatory approval for or to successfully commercialize these products, its business will be materially harmed." (S. 13).
"Microbot believes that the net cash of the Company will be sufficient to fund the Company for at least 12 months and fund operations necessary to continue development activities of the SCS and TipCAT." [...] "Raising additional capital may cause dilution to the Companys investors, restrict its operations or require it to relinquish rights to its technologies or product candidates." (S. 14).
"The market price for our Common Stock may be volatile and subject to wide fluctuations in response to factors including the following:
● actual or anticipated fluctuations in our quarterly or annual operating results;
● changes in financial or operational estimates or projections;
● conditions in markets generally;
● changes in the economic performance or market valuations of companies similar to ours;
● announcements by us or our competitors of new products, acquisitions, strategic partnerships, joint ventures or capital commitments;
● our intellectual property position; and
● general economic or political conditions in the United States, Israel or elsewhere." (S. 26).
10-K vom 2.4.2018
"Microbot has completed the development of an SCS prototype and is currently completing the safety testing, general proof of concept testing and performance testing for the device, which Microbot began in mid-2013. Microbot had a pre-submission meeting with the FDA in mid-2014. On January 27, 2017, Microbot entered into a research agreement with The Washington University in St. Louis to develop the protocol for and to execute the necessary animal study to determine the effectiveness of the Microbots SCS prototype. The initial research was completed in 2017, and a comprehensive study is expected to be completed in 2018. Upon the completion of animal studies, Microbot may conduct clinical trials if they are requested by the FDA or if Microbot decides that the data from such trials would improve the marketability of the product candidate. Microbot believes that the study results of its first generation SCS device should be submitted to the FDA by late 2018." (S. 3).
"Microbot intends to focus its marketing and sales efforts initially on pursuing collaborations with global medical device companies that have established sales and distribution networks. Microbot will seek to enter collaborations and partnerships with strategic players that offer synergies with Microbots product candidates and expertise." (S. 4).
"Microbots SCS device is universal (meaning that it is designed to be attachable to any valve on the market); therefore, Microbots initial go-to-market strategy is the development of strategic partnerships with leading global medical device companies with ready sales and distribution channels." (S. 4).
"Several academic research groups, such as at the New Jersey Institute of Technology, are currently researching sensing and obstruction-resistant catheter designs, and the Smart Sensors and Integrated Microsystems (SSIM) Program at Wayne State University has publicized that it is engaging in smart shunt development activity. However, based on its knowledge of the patented technologies, Microbot believes that these technologies are still early in the research and development cycle. The SCS also faces non-direct competition from Aqueduct Neurosciences, Inc., which is developing a non-shunt, electro-mechanical technology platform to control the draining of cerebrospinal fluid.
Microbot does not expect its SCS device to directly compete against shunt systems currently available in the market." (!!! / S.4).
"Microbot has not yet established a sales, marketing or product distribution infrastructure for its product candidates, which are still in development stages. Microbot plans to access the U.S. markets for hydrocephalus, NPH, and colonoscopy with its initial device offerings through strategic partnerships but may develop its own focused, specialized sales force or distribution channels once it has several commercialized products in its portfolio." (S. 7).
"Microbot is a development-stage medical device company and currently generates no revenue from product sales, and may never be able to commercialize SCS, TipCAT or other future product candidates, including the potential acquisition of technology from CardioSert Ltd. Microbot does not currently have the required approvals or clearances to market or test in humans SCS, TipCAT or any other future product candidates and Microbot may never receive them." [...] "Microbots business depends on the success of the SCS and the TipCAT, both of which are still in pre-clinical development. If Microbot is unable to obtain regulatory approval for or to successfully commercialize these products, its business will be materially harmed." (S. 13).
"Microbot believes that the net cash of the Company will be sufficient to fund the Company for at least 12 months and fund operations necessary to continue development activities of the SCS and TipCAT." [...] "Raising additional capital may cause dilution to the Companys investors, restrict its operations or require it to relinquish rights to its technologies or product candidates." (S. 14).
"The market price for our Common Stock may be volatile and subject to wide fluctuations in response to factors including the following:
● actual or anticipated fluctuations in our quarterly or annual operating results;
● changes in financial or operational estimates or projections;
● conditions in markets generally;
● changes in the economic performance or market valuations of companies similar to ours;
● announcements by us or our competitors of new products, acquisitions, strategic partnerships, joint ventures or capital commitments;
● our intellectual property position; and
● general economic or political conditions in the United States, Israel or elsewhere." (S. 26).
Ich habe den 10-K in der Kürze der Zeit lediglich überfliegen können.
Fakt ist: MBOT hat ein gutes, aber riskantes Konzept. MBOT leistet Pionierarbeit, wie andere Medical Companies in anderen Bereichen auch. Das kann am Ende ein riesen Ding werden ($400) oder aber auch ein Flop. Der gegenwärtige Stand, etwa des SCS, ist führend. Konkurrenz schläft zwar nicht, aber ist noch lange nicht so weit wie MBOT.
Geld ist nur in begrenztem Umfang vorhanden und wird für die Entwicklung der Produktlinie benötigt. Aufgrund dessen können noch keine Einnahmen generiert werden.
Unterm Strich wird der Kurs erst wieder nach oben gehen, wenn MBOT einen strategischen Partner ins Boot holt. Hier heißt es Geduld und Vertrauen haben und notfalls aussitzen. Kann mir vorstellen, dass ein möglicher Partner aus dem Medizinsekor erst die Ergebnisse der laufenden Studien abwartet und erst gegen Ende 2018 einsteigt. Ohne Partner wird es nicht gehen, deshalb wird im 10-K auch immer wieder die Notwendigkeit einer Kooperation betont.
A.A.o.G.
Fakt ist: MBOT hat ein gutes, aber riskantes Konzept. MBOT leistet Pionierarbeit, wie andere Medical Companies in anderen Bereichen auch. Das kann am Ende ein riesen Ding werden ($400) oder aber auch ein Flop. Der gegenwärtige Stand, etwa des SCS, ist führend. Konkurrenz schläft zwar nicht, aber ist noch lange nicht so weit wie MBOT.
Geld ist nur in begrenztem Umfang vorhanden und wird für die Entwicklung der Produktlinie benötigt. Aufgrund dessen können noch keine Einnahmen generiert werden.
Unterm Strich wird der Kurs erst wieder nach oben gehen, wenn MBOT einen strategischen Partner ins Boot holt. Hier heißt es Geduld und Vertrauen haben und notfalls aussitzen. Kann mir vorstellen, dass ein möglicher Partner aus dem Medizinsekor erst die Ergebnisse der laufenden Studien abwartet und erst gegen Ende 2018 einsteigt. Ohne Partner wird es nicht gehen, deshalb wird im 10-K auch immer wieder die Notwendigkeit einer Kooperation betont.
A.A.o.G.
Gelöschter Beitrag.
Einblenden
»
#265
Pressemitteilung v. 12.4.2018
www.microbotmedical.com/investors/...and-news/?pressid=2342234
"Die Entscheidung der Kommission beruht zum Teil auf einer erheblichen Nachfrage nach dem SCS und dessen Potenzial, neue Marktchancen zu schaffen."
Harel: "Die Anerkennung durch die Europäische Kommission und die Gewährung des Zuschusses sind ein weiterer Vertrauensbeweis für den weiteren Entwicklungsprozess unserer einzigartigen Technologie, die Größe des unerfüllten medizinischen Bedarfs, den wir lösen, und das Wertversprechen unseres SCS-Produkts. [...] Wir konzentrieren uns weiterhin auf unsere Hauptziele, um den Shareholder-Value zu steigern. Einer davon ist die Stärkung unserer Bilanz durch nicht-verwässernde Quellen, um sicherzustellen, dass wir unsere Entwicklungs- und Vermarktungspläne fehlerfrei fortsetzen. Dieser Erfolg in Verbindung mit zusätzlichen Meilensteinen, die wir in naher Zukunft erwarten, wie der Abschluss der Akquisition von CardioSert und der Abschluss der beiden laufenden Studien in Bezug auf unser führendes SCS-Produkt, wird in absehbarer Zeit für Dynamik sorgen."
"Der bewilligte Zuschuss von 50.000 kann innerhalb von sechs Monaten durch einen zusätzlichen Antrag auf bis zu 2.000.000 erhöht werden. Der Zuschuss wird für die Weiterentwicklung des Self-Cleaning Shunt (SCS) Produkts des Unternehmens verwendet. Das Unternehmen geht davon aus, dass es die erwarteten Entwicklungsmeilensteine für seine SCS erreichen wird, einschließlich der Einreichung bei der US-amerikanischen Food and Drug Administration (FDA) Ende 2018 oder Anfang 2019."
www.microbotmedical.com/investors/...and-news/?pressid=2342234
"Die Entscheidung der Kommission beruht zum Teil auf einer erheblichen Nachfrage nach dem SCS und dessen Potenzial, neue Marktchancen zu schaffen."
Harel: "Die Anerkennung durch die Europäische Kommission und die Gewährung des Zuschusses sind ein weiterer Vertrauensbeweis für den weiteren Entwicklungsprozess unserer einzigartigen Technologie, die Größe des unerfüllten medizinischen Bedarfs, den wir lösen, und das Wertversprechen unseres SCS-Produkts. [...] Wir konzentrieren uns weiterhin auf unsere Hauptziele, um den Shareholder-Value zu steigern. Einer davon ist die Stärkung unserer Bilanz durch nicht-verwässernde Quellen, um sicherzustellen, dass wir unsere Entwicklungs- und Vermarktungspläne fehlerfrei fortsetzen. Dieser Erfolg in Verbindung mit zusätzlichen Meilensteinen, die wir in naher Zukunft erwarten, wie der Abschluss der Akquisition von CardioSert und der Abschluss der beiden laufenden Studien in Bezug auf unser führendes SCS-Produkt, wird in absehbarer Zeit für Dynamik sorgen."
"Der bewilligte Zuschuss von 50.000 kann innerhalb von sechs Monaten durch einen zusätzlichen Antrag auf bis zu 2.000.000 erhöht werden. Der Zuschuss wird für die Weiterentwicklung des Self-Cleaning Shunt (SCS) Produkts des Unternehmens verwendet. Das Unternehmen geht davon aus, dass es die erwarteten Entwicklungsmeilensteine für seine SCS erreichen wird, einschließlich der Einreichung bei der US-amerikanischen Food and Drug Administration (FDA) Ende 2018 oder Anfang 2019."
Auch vor dem Hintergrund der heutigen Nachrichten glaube ich fest daran, dass sich unsere Geduld auszahlen wird!
Die Europäische Kommission hat vor allem Vertrauen - das zeigt die PM ganz deutlich. Auch in Brüssel hat man das Potenzial des Shunt Systems erkannt. Bislang hat ja nur die israelische Regierung öffentliche Gelder zur Verfügung gestellt. Insofern ist das schon ein dickes Ausrufezeichen, wenn die Kommission bereit ist, aus dem von 28 Mitgliedstaaten gespeisten Haushalt, bis zu 2 Mio Euro bereitzustellen.
Nach dem letzten 10-Q und den heutigen Nachrichten werde ich definitiv keine Shares mehr weggeben. Auch wenn sich der Kurs bis zum Jahresende auf niedrigem Niveau dahin schleppt, sind die Aussichten positiv:
1. Zusätzliche Mittel, ohne den Kurs zu verwässern.
2. Abschluss der Studien in diesem Quartal
3. Einstellung von 4-6 weiteren Mitarbeitern, die die operativen Forschungs- und Entwicklungsaktivitäten sowie die regulatorischen und klinischen Entwicklungsaktivitäten unterstützen sollen
4. Zusammenarbeit mit mehreren Krankenhäusern für klinische Studien
5. Zusammenarbeit mit einem strategischen Partner aus dem Medizinsektor
6. Einreichung Antrag FDA
Sollten sich diese Punkte bis Anfang 2019 realisieren lassen, bewegen wir uns definitiv nicht mehr im $ 1.00-Bereich!
Die Europäische Kommission hat vor allem Vertrauen - das zeigt die PM ganz deutlich. Auch in Brüssel hat man das Potenzial des Shunt Systems erkannt. Bislang hat ja nur die israelische Regierung öffentliche Gelder zur Verfügung gestellt. Insofern ist das schon ein dickes Ausrufezeichen, wenn die Kommission bereit ist, aus dem von 28 Mitgliedstaaten gespeisten Haushalt, bis zu 2 Mio Euro bereitzustellen.
Nach dem letzten 10-Q und den heutigen Nachrichten werde ich definitiv keine Shares mehr weggeben. Auch wenn sich der Kurs bis zum Jahresende auf niedrigem Niveau dahin schleppt, sind die Aussichten positiv:
1. Zusätzliche Mittel, ohne den Kurs zu verwässern.
2. Abschluss der Studien in diesem Quartal
3. Einstellung von 4-6 weiteren Mitarbeitern, die die operativen Forschungs- und Entwicklungsaktivitäten sowie die regulatorischen und klinischen Entwicklungsaktivitäten unterstützen sollen
4. Zusammenarbeit mit mehreren Krankenhäusern für klinische Studien
5. Zusammenarbeit mit einem strategischen Partner aus dem Medizinsektor
6. Einreichung Antrag FDA
Sollten sich diese Punkte bis Anfang 2019 realisieren lassen, bewegen wir uns definitiv nicht mehr im $ 1.00-Bereich!
quote.morningstar.ca/Quicktakes/owners/...SA&culture=en-CA
ich habe mir deinen Link angeschaut, die Anteile der Institionellen hat sich drastisch verringert nach dem kurzen "FlashCrash" in der USA. Komisch ist, nun steigt die Aktie stetig wieder, verrückte Welt. m.M.
ich habe mir deinen Link angeschaut, die Anteile der Institionellen hat sich drastisch verringert nach dem kurzen "FlashCrash" in der USA. Komisch ist, nun steigt die Aktie stetig wieder, verrückte Welt. m.M.
Mmmh ... eine Erklärung habe ich für diese Entwicklung auch nicht. Allerdings wurde im Nachgang des Crashs berichtet, dass sich viele institutionelle Anleger in sichere Häfen zurückziehen, aus Angst, das Blutbad könnte sich fortsetzen. Während sich andere Biotechs schnell erholt haben, dauerte es bei MBOT länger bzw. dauert es noch an. Hintergrund könnte sein, das Harel im Herbst 2017 zu forsch die Absicht kundgetan hat, einen Meilenstein im IV. Quartal erreichen zu wollen (Partnership). Das ist im allerdings (noch) nicht gelungen. Rückblickend nicht überraschend, da niemand die Katze im Sack kaufen möchte, sprich, potenzielle Partner wollen study results sehen, ehe sie einsteigen.
Diese Entwicklung sehen auch die institutionellen Anleger, die nun möglicherweise auch auf "die" großen News (Partnership, study results) warten und dann wieder einsteigen.
Bei einem Vergleich der Übersichten über die Shareholder (#170/#268) ist mir auch aufgefallen, dass Sabby endlich raus ist. Die Amis machen im US-Forum stets Sabby für massive Short-Attacken verantwortlich (Ausweislich der letzten Quartalsberichte lief wohl auch ein Rechtsstreit zwischen MBOT und Sabby.). Soll angeblich deren Geschäftsmodell sein. Mancher Anleger macht die Entscheidung über einen Einstieg sogar davon abhängig, ob Sabby an Board ist. Sollte das stimmen, kann das für uns nun nur positiv sein.
Ebenfalls positiv zu bewerten ist das weitere Engagement von MEDX. Chairman ist dort ebenfalls Harel Gadot. Bill Gates steckt dort ebenfalls drin. Als MDEX im Juni 2017 bei MBOT eingestiegen ist, betrug der Kurs der MBOT-Aktie um die $2.50 - die wissen also worauf sie sich einlassen!
Diese Entwicklung sehen auch die institutionellen Anleger, die nun möglicherweise auch auf "die" großen News (Partnership, study results) warten und dann wieder einsteigen.
Bei einem Vergleich der Übersichten über die Shareholder (#170/#268) ist mir auch aufgefallen, dass Sabby endlich raus ist. Die Amis machen im US-Forum stets Sabby für massive Short-Attacken verantwortlich (Ausweislich der letzten Quartalsberichte lief wohl auch ein Rechtsstreit zwischen MBOT und Sabby.). Soll angeblich deren Geschäftsmodell sein. Mancher Anleger macht die Entscheidung über einen Einstieg sogar davon abhängig, ob Sabby an Board ist. Sollte das stimmen, kann das für uns nun nur positiv sein.
Ebenfalls positiv zu bewerten ist das weitere Engagement von MEDX. Chairman ist dort ebenfalls Harel Gadot. Bill Gates steckt dort ebenfalls drin. Als MDEX im Juni 2017 bei MBOT eingestiegen ist, betrug der Kurs der MBOT-Aktie um die $2.50 - die wissen also worauf sie sich einlassen!
und schaue nicht mehr jeden Tag ins Depot...bin da längerfristig interessiert und habe die Zeit mitgebracht...Bin also LONG!
Eben auf der MBOT Homepage veröffentlicht:
Das Patent- und Markenamt der Vereinigten Staaten (USPTO) hat dem SCS das US Patent erteilt!!!
www.microbotmedical.com/investors/...and-news/?pressid=2348029
Das Patent- und Markenamt der Vereinigten Staaten (USPTO) hat dem SCS das US Patent erteilt!!!
www.microbotmedical.com/investors/...and-news/?pressid=2348029
Mittlerweile gehe ich auch davon aus, dass die zum Quartalsende erwarteten Studienergebnisse positiv ausfallen.
Andernfalls ergibt die vor eine Woche veröffentlichte Pressemitteilung keinen Sinn. Danach zieht MBOT in eine 6.000qm große Forschungseinrichtung um.
www.microbotmedical.com/investors/...and-news/?pressid=2346250
Bislang beschäftigt MBOT ja nur eine Handvoll Mitarbeiter. Hier ist definitiv etwas größeres im Entstehen. Ausweislich der Pressemitteilung wird die neue Anlage die gesamten israelischen Aktivitäten des Unternehmens beherbergen, darunter Ingenieure, Forscher, Hersteller, klinische und kommerzielle Teams. Der Raum umfasst auch ein Labor mit einem sauberen Bereich, um die Produktion zu ermöglichen, sowie die Entwicklung neuer Konzepte für medizinische Produkte der Mikro-Robotik zu evaluieren und zu unterstützen.
Wenn ich jetzt einmal weiter träume, würde ich sagen:
1. Studienergebnisse positiv
2. SCS für US-Markt patentiert
3. durch 1+2 kann ein weiterer Förderantrag bei der EU Kommission gestellt werden, die 2 Mio Förderung in Aussicht gestellt hat
4. die neue Einrichtung deutet daraufhin, das MBOT den SCS selbst produziert
5. durch 1+2 ist der SCS soweit validiert, das ein Partner einsteigen wird (bislang gab es nichts "handfestes")
6. Gerücht: im Gebäudekomplex, in dem die neue Forschungseinrichtung nun sitzt, unterhält auch Boston Scientific eine Niederlassung. Zufall?
Andernfalls ergibt die vor eine Woche veröffentlichte Pressemitteilung keinen Sinn. Danach zieht MBOT in eine 6.000qm große Forschungseinrichtung um.
www.microbotmedical.com/investors/...and-news/?pressid=2346250
Bislang beschäftigt MBOT ja nur eine Handvoll Mitarbeiter. Hier ist definitiv etwas größeres im Entstehen. Ausweislich der Pressemitteilung wird die neue Anlage die gesamten israelischen Aktivitäten des Unternehmens beherbergen, darunter Ingenieure, Forscher, Hersteller, klinische und kommerzielle Teams. Der Raum umfasst auch ein Labor mit einem sauberen Bereich, um die Produktion zu ermöglichen, sowie die Entwicklung neuer Konzepte für medizinische Produkte der Mikro-Robotik zu evaluieren und zu unterstützen.
Wenn ich jetzt einmal weiter träume, würde ich sagen:
1. Studienergebnisse positiv
2. SCS für US-Markt patentiert
3. durch 1+2 kann ein weiterer Förderantrag bei der EU Kommission gestellt werden, die 2 Mio Förderung in Aussicht gestellt hat
4. die neue Einrichtung deutet daraufhin, das MBOT den SCS selbst produziert
5. durch 1+2 ist der SCS soweit validiert, das ein Partner einsteigen wird (bislang gab es nichts "handfestes")
6. Gerücht: im Gebäudekomplex, in dem die neue Forschungseinrichtung nun sitzt, unterhält auch Boston Scientific eine Niederlassung. Zufall?
Geduld zahlt sich hoffentlich aus! ;-)
Es gibt keine neuen Beiträge.
|
Neueste Beiträge aus dem Microbot Medical Forum
| Wertung | Antworten | Thema | Verfasser | letzter Verfasser | letzter Beitrag | |
| 20 | ...wie meistens.. | Nobsy11 | Dölauer | 11.11.24 14:24 | ||
| 8 | 106 | StemCells ! Günstig wie nie .... | Chalifmann3 | Systema | 25.04.21 01:39 | |
| 3 | 392 | Microbot Medical übernimmt Stemcells - Neustart | Systema | Systema | 24.04.21 23:42 | |
| 5 | 63 | Stemcells aufpassen!!! | soros | snapple76 | 17.12.10 23:25 | |
| 2 | Schaut euch mal StemCells an! | Rajiva35 | Rajiva35 | 03.04.06 19:55 |