Anzeige
Meldung des Tages: Ist das DIE Rohstoff-Story des Jahres?
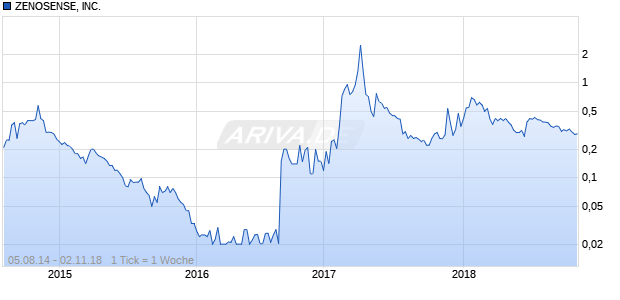
ZENOSENSE, INC.
Aktie
WKN: ISIN: US9894241069
Keine aktuellen Kursdaten verfügbar
Depot/Watchlist
Dieses Wertpapier ist nicht mehr handelbar.
Marktkapitalisierung *
-
Streubesitz
-
KGV
-
Index-Zuordnung
-
- Push
- Intraday
- 1W
- 3M
- 6M
- 1J
- 5J
- Gesamt
Werbung
Mehr Nachrichten kostenlos abonnieren
E-Mail-Adresse
Bitte überprüfe deine die E-Mail-Adresse.
Benachrichtigungen von ARIVA.DE
(Mit der Bestellung akzeptierst du die Datenschutzhinweise)
(Mit der Bestellung akzeptierst du die Datenschutzhinweise)

Vielen Dank, dass du dich für unseren Newsletter angemeldet hast. Du erhältst in Kürze eine E-Mail mit einem Aktivierungslink.
Leider können wir deine Anfrage auf diesem Weg nicht entgegennehmen.
Bitte schreibe uns an: portal.support@ariva.de
Bitte schreibe uns an: portal.support@ariva.de
Termine
Keine Termine bekannt.
Prognose & Kursziel
Keine aktuellen Prognosen oder Kursziele bekannt.
Stammdaten
| Aktientyp | Stammaktie |
Community-Beiträge zu ZENOSENSE, INC.
buran
klick und ne Stimmer spricht - das fetzt
..klick klick klick https://www.zenosense.com/ GrB
pennystockpick
Update: Notice of a patent issuance in the U.S.
VALENCIA, SPAIN--(Marketwired - Jun 15, 2017) - Zenosense, Inc. ( OTC PINK : ZENO ) ("Zenosense", the "Company"), a healthcare technology company primarily focused on the development and commercialization of MIDS Cardiac, a Point of Care ("POC") handheld device for the early detection of certain cardiac event biomarkers to significantly accelerate the triage, diagnosis, treatment and disposition of patients reporting chest pain and with suspected acute myocardial infarction (heart attack), is pleased to announce that its MIDS Medical Limited joint venture ("MML") based at Sci-Tech, Daresbury (UK), has received notice of a patent issuance in the U.S.
Our joint venture partner in MML has received a United States Patent and Trademark Office grant for the issuance of the central MIDS patent under number 9,678,064 in the U.S., namely: "Immunoassay Apparatus Incorporating Microfluidic Channel".
This is as a result of a patent application originally filed in 2011 by our MML joint venture partner. It is one of a number of applications made in commercially crucial regions for patent protection of the central MIDS detection technology.
The same MIDS patent has already been granted in China. Other applications have also successfully progressed into the national phase in the EU and India. This issuance in the U.S. indicates that these latter territories are also likely to progress to grant and, if so, will establish intellectual property protection in these key commercial regions. The MIDS patents are under license for the exclusive use by MML and will be transferred directly to MML in the event of the conclusion of a relevant commercial deal with a third party.
Carlos Gil, CEO of Zenosense Inc., commented: "This latest grant of the MIDS patent in the U.S. further validates our confidence in the ability of the MML team to deliver this exciting technology. I also expect to be able to provide a further update on development activities in the coming weeks."
hotstockpick
Update: Notice of a patent issuance in the U.S.
VALENCIA, SPAIN--(Marketwired - Jun 15, 2017) - Zenosense, Inc. ( OTC PINK : ZENO ) ("Zenosense", the "Company"), a healthcare technology company primarily focused on the development and commercialization of MIDS Cardiac™, a Point of Care ("POC") handheld device for the early detection of certain cardiac event biomarkers to significantly accelerate the triage, diagnosis, treatment and disposition of patients reporting chest pain and with suspected acute myocardial infarction (heart attack), is pleased to announce that its MIDS Medical Limited joint venture ("MML") based at Sci-Tech, Daresbury (UK), has received notice of a patent issuance in the U.S.
Our joint venture partner in MML has received a United States Patent and Trademark Office grant for the issuance of the central MIDS patent under number 9,678,064 in the U.S., namely: "Immunoassay Apparatus Incorporating Microfluidic Channel".
This is as a result of a patent application originally filed in 2011 by our MML joint venture partner. It is one of a number of applications made in commercially crucial regions for patent protection of the central MIDS detection technology.
The same MIDS patent has already been granted in China. Other applications have also successfully progressed into the national phase in the EU and India. This issuance in the U.S. indicates that these latter territories are also likely to progress to grant and, if so, will establish intellectual property protection in these key commercial regions. The MIDS patents are under license for the exclusive use by MML and will be transferred directly to MML in the event of the conclusion of a relevant commercial deal with a third party.
Carlos Gil, CEO of Zenosense Inc., commented: "This latest grant of the MIDS patent in the U.S. further validates our confidence in the ability of the MML team to deliver this exciting technology. I also expect to be able to provide a further update on development activities in the coming weeks."
hotstockpick
Zenosense
thats what I found...
ZenoSense (OTC ZENO) is rapidly becoming a major player in the medical device field, and experts are saying it is set to virtually explode onto the scene.
ZenoSense (OTC ZENO) is a company which combines industry-leading talent and stringent standards to measure success. The medical device industry took a major credibility hit with the revelations that Theranos may have mislead investors and hyped up their technology in order to grow and roll out their services. While this has been seen as a negative for the industry, ZenoSense has found a major opportunity, leading to some stunning potential upsets in the practice of using new technology to replace standard laboratory testing.
The medical device industry has always been one of the more profitable industries in the country. Combining the testing standards of pharmaceuticals, but not requiring the hundreds of millions of dollars necessary to succeed with the FDA, the profits generated by solid technology and corporate transparency have yielded amazing returns. ZenoSense (OTC ZENO) makes sure to adhere to both principles as its core philosophy. While most medical technology companies go public only as a way to help investors feel more comfortable with the liquidity of their investment, ZenoSense does it in order to be able to adhere to the rigid standards of the SEC right from the beginning. It is more than faith in the technology, it is faith in the company as a whole -- with a transparent mission, ZenoSense offers an opportunity that its investors can feel comfortable with, and innovative technology that is changing the industry.
There have been more than a few companies that have ventured into the field of testing technology, but few have received the results ZenoSense (OTC ZENO) has in such a short period of time. The core focus of ZenoSense has been through a joint venture with MIDS Medical focusing on a product called MIDS Cardiac™. A handheld device which is being developed to provide rapid testing of cardiac markers for use at the Point of Care, MIDS Cardiac could give doctors the ability to provide their patients with diagnostic results equal or superior to laboratory tests. This not only allows the results to be able to aide in treatment almost in real time, but allows patients to avoid the deadly delays that even a day of waiting can cause. ZenoSense can confidently say that MIDS Cardiac™ can revolutionize the diagnosis and management of suspected Acute Myocardial Infarction (AMI), a major cardiac condition.
ZenoSense (OTC ZENO) isn't just tearing up the markets, but the industry as a whole as well. The medical device industry, as measured by the Dow Jones Medical Equipment Index, with combined sales in the trillions of dollars, has consistently provided returns to investors above 10% annually since it began being measured. It has solidly outperformed the S&P Index in every year it was measured since the index's inception in 2006.
To see the opportunity provided by ZenoSense (OTC ZENO), all one has to do is dive even further into the industry. According to a 2015 report sponsored by the American Heart Association, 85.6 million people suffer from cardiac diseases in the United States alone. With the population aging and the Baby Boomer generation retiring, the growth in obesity rates and other factors is only going to increase. Experts have also cautioned that with the addition of more than 20 million people to the health insurance rolls since the rollout of the Affordable Care Act, there is also a further strain on the amount of doctors and laboratory services available. The medical device industry has been there consistently to pick up the slack, by shortening the time period between testing and diagnosis, reducing costs and providing better outcomes to the public.
Additionally, there's a fear that the tax on the industry from the Affordable Care Act would stifle innovation and reduce the ability of the industry to grow as it has. In a sign of stunning bipartisanship, both parties in the United States passed a suspension of that tax for two years in 2015. There is talk about the tax facing certain repeal under the current administration, but even if it comes back, it is a stronger incentive to get into the industry now as there is still more than a year left before it is slated to return.
ZenoSense (OTC ZENO) has also made sure to use its current resources to ensure that its MIDS Medical team is made up of some of the best and brightest staff in the world. With more than thirty years' experience in magnetic measurement and sensor design, and a focus on nanoparticle applications and the highest industry experts in micro-magnetic and luminescence techniques, it stands alone at the top of the field. Strengthening the team even further is recent news of a contract to work with Future Diagnostics who have developed assays for several of the world's largest multi-national diagnostic companies and highly specialist in modern cardiac assays.
Jetzt anmelden und diskutieren
Registrieren
Login
Zum Thread wechseln Häufig gestellte Fragen zur ZENOSENSE, INC. Aktie und zum ZENOSENSE, INC. Kurs
Nein, ZENOSENSE, INC. zahlt keine Dividenden.