Jun 14, 2010
ir.cytoritx.com/InvestorRelations/...tail.cfm?ReleaseID=479210
H. D. Thoreau
|
SAN DIEGO--(BUSINESS WIRE)--Cytori Therapeutics (NASDAQ: CYTX - News) has received European (CE Mark) approval for the PureGraft™ 250/PURE System for autologous fat grafting procedures, allowing Cytori to immediately commercialize the PureGraft™ product line to physicians in Europe. PureGraft™ will be sold as both a standalone product and as a complement to Cytori’s Celution® 800/CRS System in Europe.
The PureGraft™ System is an optimized tissue processing technology that is positioned at the forefront of the emerging fat grafting trend in the cosmetic and reconstructive surgery market. PureGraft™ replaces current non-standardized methods of graft preparation. Used independently, PureGraft™ rapidly and reliably produces optimal graft tissue for use in autologous fat grafting procedures. In combination with the Celution® 800/CRS, PureGraft™ lowers processing times and increases processing volumes, improving the utility and efficiency of Cytori’s core product for soft tissue applications.
“The addition of PureGraft™ to our product portfolio allows us to offer a broad spectrum of autologous fat grafting technologies in Europe. In addition to Celution® System, which enables cell-enriched fat grafting procedures, PureGraft™ fulfills the need for high quality, sterile tissue for fat grafting procedures, including body contouring,” said Eric Daniels, M.D., Cytori’s Managing Director in Europe and the Middle East.
The PureGraft™ System sets a new standard for fat graft processing with its membrane-based tissue filtration combined with speed, simplicity, safety and precision. The PureGraft™ technology takes only 15 minutes to purify a fat graft ranging from 50 to 250 mL, removing excess and unwanted fluid, lipid, blood cells and debris in a controllable manner. The consumable-based system, used within the sterile field, “dialyzes” off everything but the purified fat tissue without centrifugation or other methods. The ease of use and simplicity of this innovative system sets it apart from other traditional fat grafting methods.
The PureGraft™ 250/PURE System received 510(k) marketing clearance for aesthetic body contouring from the U.S. FDA in January of this year. The product was formally launched at the meeting of the American Society of Aesthetic Plastic Surgeons in April 2010. In addition, Cytori received a Canadian medical device license for PureGraft™ in March 2010.
For more information, visit www.puregraft.com.
About Cytori ....
http://finance.yahoo.com/news/...stem-bw-2252723998.html?x=0&.v=1
SmarTrend is bullish on shares of Cytori Therapeutics
Top Biotechnology Small Caps of the Week
Cytori Therapeutics, Inc. which belongs to the Biotechnology sector has a float of approximately 42.44M shares and approximately 50.51M shares outstanding. It
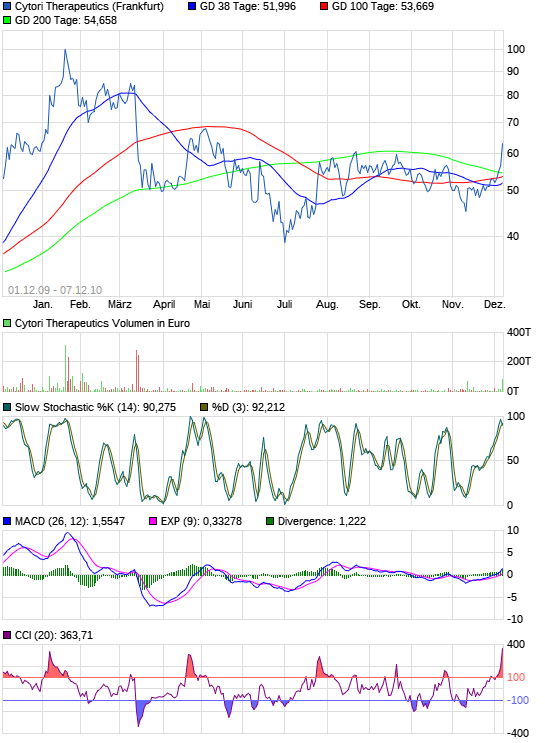
Cytori approved to initiate Europe stem & regenerative cell heart attack trial
|
| Wertung | Antworten | Thema | Verfasser | letzter Verfasser | letzter Beitrag | |
| 2 | 122 | Cytx | chacha1 | mike64 | 25.04.21 01:20 | |
| 2 | Cytori Therapeutics: Berichte Q1 2018 | Baroh | Kursverlauf_ | 29.05.18 09:42 | ||
| 7 | 338 | Cytori Therapeutics | LarsvomMars | JoeUp | 02.03.15 13:05 | |
| 11 | 311 | Macropore biosurgery: Charttechnischer Kauf | ecki | kinu | 26.05.11 20:04 |