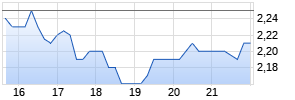
Trovagene Announces Adjournment of Special Meeting of Stockholders Until February 12, 2019
PR Newswire
SAN DIEGO, Jan. 15, 2019
SAN DIEGO, Jan. 15, 2019 /PRNewswire/ -- Trovagene, Inc. (Nasdaq: TROV), a clinical-stage oncology therapeutics company, taking a precision cancer medicine approach to develop drugs that target cell division (mitosis) for the treatment of leukemias, lymphomas and solid tumor cancers, announced today that its Special Meeting of Stockholders ("Special Meeting"), scheduled for Tuesday, January 15, 2019, has been adjourned. At the meeting, it was deemed that the holders of a sufficient number of Trovagene's outstanding common stock have not yet submitted proxies to indicate how their shares should be voted and additional time was needed to collect the required votes. The Special Meeting will resume at 9:00 a.m. PST on February 12, 2019 at the company's offices located at 11055 Flintkote Avenue, San Diego, California 92121.

The company will continue to solicit proxies from stockholders during the period of adjournment. Only stockholders of record on the record date of December 14, 2018 are entitled and are being requested to vote. No further action is required by any stockholder who has submitted his or her proxy card. The company's Board of Directors and management respectfully request all such holders as of the record date who have not yet voted their shares to do so by 11:59 p.m. PST on Monday, February 11, 2019.
How to Vote
For assistance in voting your shares or for general inquiries please contact our proxy advisor
Kingsdale Advisors
(866) 851-2484 (toll free)
contactus@kingsdaleadvisors.com
About Trovagene, Inc.
Trovagene is a clinical-stage, oncology therapeutics company, taking a precision medicine approach to develop drugs that target mitosis (cell division) to treat various types of cancer, including leukemias, lymphomas and solid tumors. Trovagene has intellectual property and proprietary technology that enables the Company to analyze circulating tumor DNA (ctDNA) and clinically actionable markers to identify patients most likely to respond to specific cancer therapies. Trovagene plans to continue to vertically integrate its tumor genomics technology with the development of targeted cancer therapeutics. For more information, please visit www.trovageneoncology.com.
Forward-Looking Statements
Certain statements in this press release are forward-looking within the meaning of the Private Securities Litigation Reform Act of 1995. These statements may be identified by the use of words such as "anticipate," "believe," "forecast," "estimated" and "intend" or other similar terms or expressions that concern Trovagene's expectations, strategy, plans or intentions. These forward-looking statements are based on Trovagene's current expectations and actual results could differ materially. There are a number of factors that could cause actual events to differ materially from those indicated by such forward-looking statements. These factors include, but are not limited to, our need for additional financing; our ability to continue as a going concern; clinical trials involve a lengthy and expensive process with an uncertain outcome, and results of earlier studies and trials may not be predictive of future trial results; our clinical trials may be suspended or discontinued due to unexpected side effects or other safety risks that could preclude approval of our product candidates; uncertainties of government or third party payer reimbursement; dependence on key personnel; limited experience in marketing and sales; substantial competition; uncertainties of patent protection and litigation; dependence upon third parties; our ability to develop tests, kits and systems and the success of those products; regulatory, financial and business risks related to our international expansion and risks related to failure to obtain FDA clearances or approvals and noncompliance with FDA regulations. There are no guarantees that any of our technology or products will be utilized or prove to be commercially successful. Additionally, there are no guarantees that future clinical trials will be completed or successful or that any precision medicine therapeutics will receive regulatory approval for any indication or prove to be commercially successful. Investors should read the risk factors set forth in Trovagene's Form 10-K for the year ended December 31, 2017, and other periodic reports filed with the Securities and Exchange Commission. While the list of factors presented here is considered representative, no such list should be considered to be a complete statement of all potential risks and uncertainties. Unlisted factors may present significant additional obstacles to the realization of forward-looking statements. Forward-looking statements included herein are made as of the date hereof, and Trovagene does not undertake any obligation to update publicly such statements to reflect subsequent events or circumstances.
Important Information
In connection with the solicitation of proxies, on December 18, 2018, Trovagene, Inc. filed a definitive proxy statement with the Securities and Exchange Commission (the "SEC") in connection with the Company's Special Meeting. TROVAGENE'S STOCKHOLDERS ARE STRONGLY ADVISED TO READ THE DEFINITIVE PROXY MATERIALS AND ANY OTHER RELEVANT SOLICITATION MATERIALS FILED BY TROVAGENE WITH THE SEC BEFORE MAKING ANY VOTING OR INVESTMENT DECISION BECAUSE THESE DOCUMENTS CONTAIN IMPORTANT INFORMATION. The Company's proxy statement and any other materials filed by the Company with the SEC can be obtained free of charge at the SEC's web site at www.sec.gov. The Company's definitive proxy materials are also available for free from Trovagene at www.trovagene.com or by writing to Trovagene at 11055 Flintkote Avenue, San Diego, California 92121, Attention: Investor Relations. The contents of the websites referenced above are not deemed to be incorporated by reference into the proxy statement. Trovagene, Inc. and its directors and executive officers may be deemed to be participants in the solicitation of proxies from the stockholders of Trovagene in connection with the Company's Special Meeting of Stockholders. Information concerning the interests of participants in the solicitation of proxies is included in the definitive proxy statement filed by Trovagene with the SEC on December 18, 2018 in connection with its Special Meeting of Stockholders.
Trovagene Contact:
Vicki Kelemen
VP, Clinical Development
858-952-7652
vkelemen@trovagene.com
![]() View original content to download multimedia:http://www.prnewswire.com/news-releases/trovagene-announces-adjournment-of-special-meeting-of-stockholders-until-february-12-2019-300778907.html
View original content to download multimedia:http://www.prnewswire.com/news-releases/trovagene-announces-adjournment-of-special-meeting-of-stockholders-until-february-12-2019-300778907.html
SOURCE Trovagene, Inc.

Mehr Nachrichten zur Cardiff Oncology Aktie kostenlos abonnieren
(Mit der Bestellung akzeptierst du die Datenschutzhinweise)

Hinweis: ARIVA.DE veröffentlicht in dieser Rubrik Analysen, Kolumnen und Nachrichten aus verschiedenen Quellen. Die ARIVA.DE AG ist nicht verantwortlich für Inhalte, die erkennbar von Dritten in den „News“-Bereich dieser Webseite eingestellt worden sind, und macht sich diese nicht zu Eigen. Diese Inhalte sind insbesondere durch eine entsprechende „von“-Kennzeichnung unterhalb der Artikelüberschrift und/oder durch den Link „Um den vollständigen Artikel zu lesen, klicken Sie bitte hier.“ erkennbar; verantwortlich für diese Inhalte ist allein der genannte Dritte.