
Pfenex to Regain Full Rights to PF582 and Announces Phase 1/2 Results
PR Newswire
SAN DIEGO, Aug. 8, 2016
SAN DIEGO, Aug. 8, 2016 /PRNewswire/ -- Pfenex Inc. (NYSE MKT: PFNX) announced today that the company will regain the full rights to PF582, a biosimilar candidate to Lucentis®, following our partner's strategic review of the current therapeutic focus of its biosimilar pipeline.
"With Pfenex regaining the rights to PF582 we are announcing the PF582 phase 1/2 safety and efficacy data which we believe highlights the significant value of the program," stated Bertrand C. Liang, chief executive officer of Pfenex. "We will consider strategic options for PF582 following the expeditious transition of the full development program back to Pfenex. Pfenex's development capabilities, leveraging our Pfenex Expression Technology® platform, has allowed us to advance a diverse portfolio of product candidates, including PF582, which we believe will create significant value for our shareholders. We look forward to providing key updates on our pipeline progress throughout the year."
Phase 1/2 PF582 Results
Pfenex enrolled a total of 25 VEGF-inhibitor naïve patients with neovascular age-related macular degeneration (AMD) in the PF582 phase 1/2 trial (13 received PF582, including 1 sentinel patient who received open label PF582, 12 received Lucentis). All patients received 3 monthly intravitreal injections. The primary endpoint of the study was safety and tolerability of PF582 compared to that of Lucentis in patients with neovascular AMD.
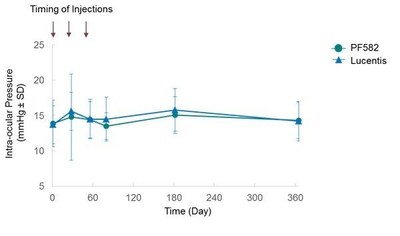
With respect to safety, there were no meaningful differences in intra-ocular pressure between PF582 and Lucentis at any of the timepoints (Figure 1). Additionally, there were no imbalances in local or systemic adverse events and no unexpected safety or tolerability findings in the population studied.
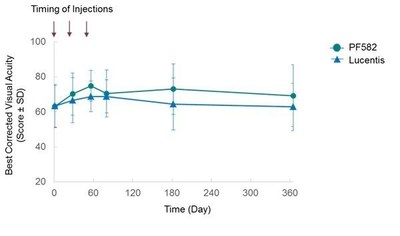
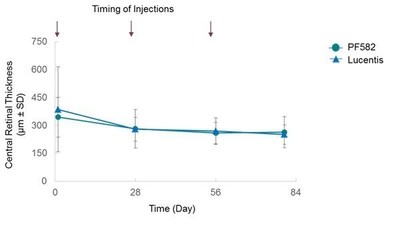
The efficacy and pharmacodynamic results indicated that there were no meaningful differences in best corrected visual acuity (Figure 2) and the decreases in central retinal thickness (Figure 3) between PF582 and Lucentis at any of the timepoints were also similar.
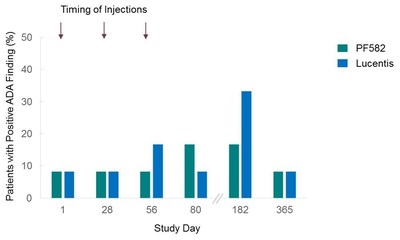
The immunogenicity results (Figure 4) showed comparable anti-drug antibody findings between PF582 and Lucentis throughout the three month study period.
This first-in-human study met its primary objective of demonstrating similar safety and tolerability between PF582 and Lucentis. Additionally, it demonstrated consistent pharmacological activity between PF582 and Lucentis.
Cautionary Note Regarding Forward-Looking Statement
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Forward-looking statements generally relate to future events or Pfenex's future financial or operating performance. In some cases, you can identify forward-looking statements because they contain words such as "may," "will," "should," "expects," "plans," "anticipates," "could," "intends," "target," "projects," "contemplates," "believes," "estimates," "predicts," "potential" or "continue" or the negative of these words or other similar terms or expressions that concern Pfenex's expectations, strategy, plans or intentions. Forward-looking statements in this press release include, but are not limited to, statements regarding the return of all rights to PF582 to Pfenex; the potential value of PF582; the ability to create significant value for shareholders; Pfenex's expectation to pursue strategic options for PF582; the future potential of Pfenex's product candidates, including future plans to develop, manufacture and commercialize its product candidates, Pfenex's expectations regarding the timing of additional clinical trials and the types of future clinical trials for its product candidates, including PF582 and its other product candidate.; Pfenex's expectations and beliefs regarding these matters may not materialize, and actual results in future periods are subject to risks and uncertainties that could cause actual results to differ materially from those projected. Actual results may differ materially from those indicated by these forward-looking statements as a result of the uncertainties inherent in the clinical drug development process, including, without limitation, Pfenex's ability to successfully demonstrate the efficacy and safety of its product candidates; the pre-clinical and clinical results for its product candidates, which may not support further development of product candidates or may require Pfenex to conduct additional clinical trials or modify ongoing clinical trials or regulatory pathways; challenges related to commencement, patient enrollment, completion, and analysis of clinical trials; difficulties in achieving and demonstrating biosimilarity in formulations; Pfenex's ability to manage operating expenses; Pfenex's ability to obtain additional funding to support its business activities and establish and maintain strategic business alliances and new business initiatives; Pfenex's dependence on third parties for development, manufacture, marketing, sales and distribution of products; unexpected expenditures; and difficulties in obtaining and maintaining intellectual property protection for its product candidates. Information on these and additional risks, uncertainties, and other information affecting Pfenex's business and operating results is contained in Pfenex's Annual Report on Form 10-K for the year ended December 31, 2015 and in Pfenex's subsequent reports on Form 10-Q and Form 8-K, filed with the Securities and Exchange Commission. Additional information will also be set forth in Pfenex's Quarterly Report on Form 10-Q for the period ended June 30, 2016 to be filed with the Securities and Exchange Commission. The forward-looking statements in this press release are based on information available to Pfenex as of the date hereof, and Pfenex disclaims any obligation to update any forward-looking statements, except as required by law.
Pfenex investors and others should note that we announce material information to the public about the Company through a variety of means, including our website (http://www.pfenex.com/), our investor relations website (http://pfenex.investorroom.com/), press releases, SEC filings, public conference calls, corporate Twitter account (https://twitter.com/pfenex), Facebook page (https://www.facebook.com/Pfenex-Inc-105908276167776/timeline/), and LinkedIn page (https://www.linkedin.com/company/pfenex-inc) in order to achieve broad, non-exclusionary distribution of information to the public and to comply with our disclosure obligations under Regulation FD. We encourage our investors and others to monitor and review the information we make public in these locations as such information could be deemed to be material information. Please note that this list may be updated from time to time.
About Pfenex Inc.
Pfenex Inc. is a clinical-stage biotechnology company engaged in the development of biosimilar therapeutics and high-value and difficult to manufacture proteins. The company's lead product candidate is PF582, a biosimilar candidate to Lucentis (ranibizumab), for the potential treatment of patients with retinal diseases. Pfenex has leveraged its Pfēnex Expression Technology® platform to build a pipeline of product candidates and preclinical products under development including other biosimilars, as well as vaccines, therapeutic equivalents to reference listed drug products, and next generation biologics.

Photo - http://photos.prnewswire.com/prnh/20160806/396195
Photo - http://photos.prnewswire.com/prnh/20160806/396196
Photo - http://photos.prnewswire.com/prnh/20160806/396197
Photo - http://photos.prnewswire.com/prnh/20160806/396198
Logo - http://photos.prnewswire.com/prnh/20140715/127348
To view the original version on PR Newswire, visit:http://www.prnewswire.com/news-releases/pfenex-to-regain-full-rights-to-pf582-and-announces-phase-12-results-300310159.html
SOURCE Pfenex Inc.

Mehr Nachrichten zur Pfenex Aktie kostenlos abonnieren
(Mit der Bestellung akzeptierst du die Datenschutzhinweise)

Hinweis: ARIVA.DE veröffentlicht in dieser Rubrik Analysen, Kolumnen und Nachrichten aus verschiedenen Quellen. Die ARIVA.DE AG ist nicht verantwortlich für Inhalte, die erkennbar von Dritten in den „News“-Bereich dieser Webseite eingestellt worden sind, und macht sich diese nicht zu Eigen. Diese Inhalte sind insbesondere durch eine entsprechende „von“-Kennzeichnung unterhalb der Artikelüberschrift und/oder durch den Link „Um den vollständigen Artikel zu lesen, klicken Sie bitte hier.“ erkennbar; verantwortlich für diese Inhalte ist allein der genannte Dritte.




