
ResMed Introduces AirFit™ N20 and AirFit F20 CPAP Masks: A New Standard for Fit and Comfort
PR Newswire
ATLANTA, Nov. 1, 2016
ATLANTA, Nov. 1, 2016 /PRNewswire/ -- ResMed (NYSE:RMD) today introduced the AirFit N20 nasal mask and AirFit F20 full face mask for positive airway pressure (PAP) treatment of sleep apnea. Loaded with innovations designed to improve mask fit, comfort and ease of use, the new-generation ResMed masks are the result of more than three years of research and development.
The AirFit N20 and AirFit F20 feature ResMed's innovative new InfinitySeal silicone cushion that adapts to the unique facial contours of each patient to increase comfort, improve fit and reduce leakage for maximum treatment efficacy. Hard-to-fit masks affect sleep specialists and patients alike, so with the new AirFit 20 series, ResMed has taken one of its most popular mask features – magnetic clips, which make the headgear easier to get on and off – and introduced them on its full face model.
Testing shows the AirFit N20 fit an astonishing 99 percent of patients tested, and the AirFit F20 fit an impressive 97 percent – regardless of facial structure, gender or age.1–5
"These new masks address two of the biggest catalysts for effective sleep apnea treatment: fit and comfort," said ResMed CEO Mick Farrell. "Patients rate mask comfort as the number one reason that can help them stay on therapy. Not only are the F20 and N20 our most comfortable masks ever, they're easier and faster for clinicians to fit on patients the first time, helping more people suffering from sleep apnea adhere to this life-changing therapy."
The masks are part of ResMed's Air Solutions connected care portfolio, complementing its best-selling, cloud-enabled Air10TM flow generator devices and myAirTM and AirViewTM monitoring tools that are proven to help improve patients' CPAP use.
Availability
The new ResMed AirFit 20 masks will be shown publicly for the first time in the U.S. at the Medtrade Fall medical industry conference in Atlanta, October 31–November 3, 2016. Visit ResMed's Booth #1511.
ResMed plans to make the AirFit F20 and AirFit N20 available in the U.S. this quarter. The launch is subject to clearance by the U.S. Food and Drug Administration. Both masks are currently being rolled out in EMEA and Asia-Pacific.
ARIVA.DE Börsen-Geflüster
Weiter aufwärts?
| Kurzfristig positionieren in ResMed | ||
|
HS3LEH
| Ask: 1,35 | Hebel: 7,74 |
| mit moderatem Hebel |
Zum Produkt
| |

Kurse
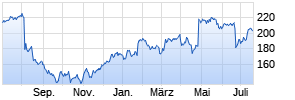 |
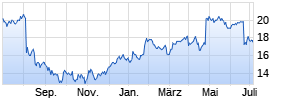 |
About ResMed
ResMed (NYSE:RMD) changes lives with award-winning medical devices and cutting-edge cloud-based software applications that better diagnose, treat and manage sleep apnea, chronic obstructive pulmonary disease (COPD) and other chronic diseases. ResMed is a global leader in connected care, with more than 2 million patients remotely monitored every day. Our 5,000-strong team is committed to creating the world's best tech-driven medical device company – improving quality of life, reducing the impact of chronic disease, and saving healthcare costs in more than 100 countries.
ResMed.com | Facebook | Twitter | LinkedIn
- ResMed internal study of 22 existing ResMed patients, conducted between April 26–May 27, 2016, comparing the market-leading mask with AirFit F20. Preliminary patient study – data on file; ID A3810791.
- ResMed AirFit F20 internal fitting study of 27 ResMed and non-ResMed patients, conducted between March 30–April 04, 2016. Preliminary patient study – data on file; ID A3751086.
- ResMed AirFit F20 internal international fitting study of 34 ResMed and non-ResMed patients, conducted between April 11–15, 2016. Preliminary patient study – data on file; ID A3774922.
- ResMed AirFit F20 internal international fitting study of 90 ResMed and non-ResMed patients, conducted between June 6–22, 2016. Preliminary patient study – data on file; ID A3830701
- ResMed AirFit N20 internal global fitting study of 159 existing ResMed patients, conducted November 12, 2015; ID A3697629.
| Contacts: | |
| For News Media | For Investors |
| Jayme Rubenstein | Agnes Lee |
| Public Relations Manager | Senior Director, Investor Relations |
| O: 858-836-6798 | O: 858-836-5971 |
| | |

Logo - http://photos.prnewswire.com/prnh/20140310/LA79234LOGO-a
To view the original version on PR Newswire, visit:http://www.prnewswire.com/news-releases/resmed-introduces-airfit-n20-and-airfit-f20-cpap-masks-a-new-standard-for-fit-and-comfort-300354561.html
SOURCE ResMed Inc.

Mehr Nachrichten zur ResMed Aktie kostenlos abonnieren
(Mit der Bestellung akzeptierst du die Datenschutzhinweise)

Hinweis: ARIVA.DE veröffentlicht in dieser Rubrik Analysen, Kolumnen und Nachrichten aus verschiedenen Quellen. Die ARIVA.DE AG ist nicht verantwortlich für Inhalte, die erkennbar von Dritten in den „News“-Bereich dieser Webseite eingestellt worden sind, und macht sich diese nicht zu Eigen. Diese Inhalte sind insbesondere durch eine entsprechende „von“-Kennzeichnung unterhalb der Artikelüberschrift und/oder durch den Link „Um den vollständigen Artikel zu lesen, klicken Sie bitte hier.“ erkennbar; verantwortlich für diese Inhalte ist allein der genannte Dritte.



